| Journal of Endocrinology and Metabolism, ISSN 1923-2861 print, 1923-287X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Endocrinol Metab and Elmer Press Inc |
| Journal website https://jem.elmerpub.com |
Review
Volume 15, Number 4, October 2025, pages 119-129
Mesenchymal Stem Cell in Thyroid Disorders: Hype or Hope?
Yan Fen Liua, Xue Yong Loua, b
aDepartment of Endocrinology, Affiliated Jinhua Hospital, Zhejiang University School of Medicine, Jinhua 321000, China
bCorresponding Author: Xue Yong Lou, Department of Endocrinology, Affiliated Jinhua Hospital, Zhejiang University School of Medicine, Jinhua 321000, China
Manuscript submitted July 19, 2025, accepted September 16, 2025, published online October 6, 2025
Short title: Mesenchymal Stem Cell in Thyroid Disorders
doi: https://doi.org/10.14740/jem1550
- Abstract
- Introduction
- Mechanisms of Action: Differentiation and Functional Restoration
- Mechanisms of Action: Immunomodulation and Microenvironment Regulation
- Application in Thyroid Nodules and Cancer
- Application in Autoimmune Thyroid Disorders
- Clinical Translation Challenges and Future Directions
- References
| Abstract | ▴Top |
Thyroid disorders, including hypothyroidism, hyperthyroidism, thyroid cancer, and nodular disease, represent a substantial global health burden. Current therapeutic options are often inadequate, frequently necessitating lifelong hormone replacement or causing irreversible tissue damage. Mesenchymal stem cells (MSCs) have emerged as a promising therapeutic platform due to their dual capacity to differentiate into thyroid-like cells and modulate immune dysregulation. This review provides a critical examination of preclinical evidence supporting MSC-based interventions for thyroid disorders. We analyzed studies demonstrating MSC potential in restoring thyroid hormone production via thyrocyte regeneration, rebalancing T-helper 17 (Th17)/regulatory T cell (Treg) cell ratios in autoimmune thyroiditis, and inhibiting tumor progression through paracrine mechanisms. Key challenges, such as tumorigenesis risk from MSC-tumor interactions, source variability, regulatory complexities for standardization, and unresolved issues in differentiation protocols and safety biomarkers, are systematically evaluated. The primary purpose of this review was to assess the therapeutic potential and limitations of MSC-based strategies for treating thyroid disorders. The review emphasizes the critical need for the development of robust translational frameworks that address existing obstacles, including the refinement of differentiation protocols, establishment of safety biomarkers, and exploration of engineered MSC-derived extracellular vesicles with targeted therapies, to bridge the gap between preclinical promise and clinical application.
Keywords: Mesenchymal stem cell; Thyroid diseases; Hypothyroidism; Thyroid nodules; Immune dysfunction modulation
| Introduction | ▴Top |
The thyroid gland, consisting of two connected lobes, is one of the largest endocrine glands in the human body, weighing 20 - 30 g in adults [1]. Thyroid lesions are often found on the gland, with a prevalence of 4-7%. Most of them are asymptomatic, and thyroid hormone (TH) secretion is normal [2]. Thyroid disorders are a common form of endocrine dysfunction, encompassing conditions such as hyperthyroidism (1.22%), hypothyroidism (13.95%), autoimmune thyroiditis (14.19%), thyroid nodules (20.43%), thyroid eye disease, and thyroid cancer [3-6]. Although conventional therapies (such as levothyroxine and radioactive iodine) have advanced, they do not target the underlying tissue damage or immune dysregulation, underscoring the need for regenerative approaches. Mesenchymal stem cells (MSCs), multipotent stromal cells with self-renewal and immunomodulatory properties, present a dual therapeutic potential by differentiating into thyroid-like cells and addressing autoimmune inflammation [7].
Despite the rising prevalence of thyroid disorders and the introduction of various pharmacological, non-pharmacological, and combined interventions, no comprehensive therapeutic strategy has yet been established to completely alleviate the symptoms. Moreover, concerns are increasing about the potential risks associated with the simultaneous use of multiple medications. Understanding the complex pathology of thyroid disorders is crucial to gaining deeper insights that can inform future research and improve treatment approaches. Traditional treatments typically target specific syndromic components of these disorders.
A novel approach has emerged in mesenchymal stem cell-derived extracellular vesicle (MSC-EV), therapy, offering a transformative shift in perspective. Stem cells are found in both embryonic and adult forms. The MSCs are multipotent cells that differentiate into mesodermal tissues. Initially isolated from bone marrow (bone marrow-derived mesenchymal stem cells (BM-MSCs)), MSCs can also be derived from other sources such as adipose tissue, dental pulp, umbilical cord blood, and placenta. Their remarkable ability to proliferate and immunosuppressive properties make MSCs promising candidates for clinical applications in treating various diseases. Furthermore, they hold significant potential in regenerative medicine [8]. This review focuses exclusively on stem cell applications in thyroid disorders.
| Mechanisms of Action: Differentiation and Functional Restoration | ▴Top |
Mechanisms of stem cell therapy for thyroid disease
Stem cell therapy for thyroid disorders leverages the capacity of MSCs to differentiate into thyroid-like cells, promoting tissue repair and functional restoration. This therapeutic process involves a series of steps - proliferation, migration, and differentiation - regulated by the expression of thyroid-specific genes. MSCs from sources such as bone marrow and adipose tissue possess a limited differentiation potential but offer remarkable advantages, including immune compatibility and anti-inflammatory effects. The differentiation of MSCs into thyroid follicular cells follows a stepwise process involving proliferation, migration, and the expression of thyroid-specific markers such as paired box gene 8 (PAX8), NK2 homeobox1 (NKX2-1), and thyroid-stimulating hormone receptor (TSHR). Although the expression of thyroid-specific markers (e.g., PAX8, NKX2-1) is a crucial first step, the ultimate proof of function is the production of THs. While direct in vitro measurement of T3/T4 secretion is not commonly reported in the MSC literature, functional recovery has been convincingly demonstrated in animal models. For instance, the restoration of normal serum T4 and thyroid-stimulating hormone (TSH) levels in hypothyroid rats following transplantation has been reported. In one key study, Sca-1+ mesenchymal cells were identified as progenitor cells contributing to the proliferation of follicular cells during thyroid regeneration [9]. Similarly, another study demonstrated that BM-MSCs differentiated into thyrocyte-like cells in vitro via TSH stimulation, restoring TH levels in hypothyroid rats [10]. This key in vivo finding provides the most direct evidence of functional restoration, proving that the transplanted cells were not only present but also capable of normalizing systemic thyroid function. Figure 1 highlights the key signaling pathways involved in differentiating MSCs into thyroid-like cells, as supported by findings from these studies. The above findings provides strong in vivo evidence that the differentiated cells possess hormonal activity. Future studies should incorporate direct functional assays to further validate these findings.
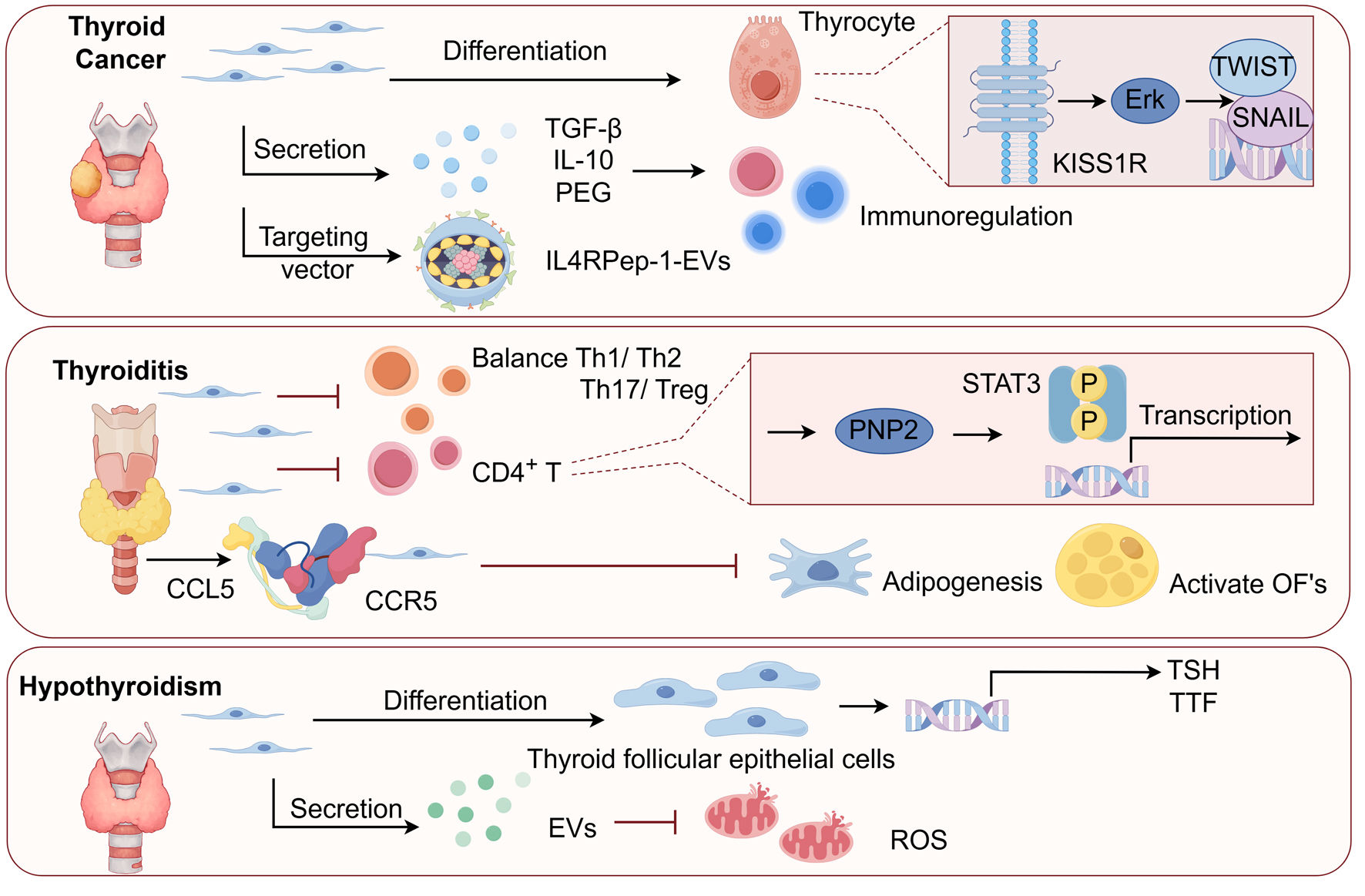 Click for large image | Figure 1. The role and potential of mesenchymal stem cells (MSCs) in thyroid disease therapy. MSCs have been shown to migrate to damaged thyroid tissue, modulate immune responses, and differentiate into thyroid follicular cells, offering potential therapeutic avenues for conditions such as hypothyroidism and autoimmune thyroiditis. In thyroid cancer, MSCs reveal a complex role, with studies suggesting both tumor-promoting activities and therapeutic potential, particularly through gene delivery systems. TGF-β1: tumor growth factor-β1; PGE2: prostaglandin E2; Th: T-helper; IL: interleukin; EVs: extracellular vesicles; ROS: reactive oxygen species; TSH: thyroid-stimulating hormone; TTF: thyroid transcription factor; CCL5: C-C motif chemokine ligand 5; CCR5: C-C motif chemokine receptor 5; OF: orbital fibroblast. |
Stem cell differentiation of thyroid-like cells
The differentiation of stem cells into thyroid-like cells involves a coordinated sequence of proliferation, migration, and maturation into functionally specialized cells with thyroid-specific roles. This complex process requires tightly regulated mechanisms to ensure the proper expression of thyroid-specific genes and proteins. While MSCs are constrained to differentiate into specialized cell types, they remain a valuable source for regenerative applications [11]. Moreover, research on the role of BM-MSCs in thyroid tissue regeneration remains limited. The thyroid gland comprises two primary cell types: follicular and parafollicular (C) cells, with most follicular cells forming during early gland development. However, the origin of the follicular cells that proliferate after partial thyroidectomy is still poorly understood. Okamoto et al [12] employed long-label retention analysis to trace the genetic lineage of newly proliferating follicular cells. The study demonstrated that these cells originate from mesenchymal cells expressing stem cell antigen 1. Furthermore, epithelial-mesenchymal transition plays a pivotal role in developing C-cell-derived carcinoma, particularly medullary thyroid carcinoma [13].
In a diabetic model, rat BM-MSCs were found to reduce reactive oxygen species (ROSs) levels and help preserve thyroid function. Intravenous administration of these MSCs also allowed for the investigation of pancreatic abnormalities associated with hypothyroidism. Although MSCs possess a lower regenerative potential compared to embryonic stem cells (ESCs), they offer distinct therapeutic advantages. MSCs mainly express low levels of major histocompatibility complex (MHC) antigens on their surface, improving their compatibility with the immune system [14]. Thirdly, MSCs can migrate to injured tissues or organs and exert pro-inflammatory or anti-inflammatory effects, depending on the local microenvironment.
Because of their unique properties, MSCs are regarded as highly promising candidates for treating both immune-related and non-immune diseases [15]. Stem cells, especially MSCs, can differentiate into specific cell types, including thyroid follicular cells while demonstrating anti-inflammatory and anti-cancer properties. These characteristics highlight their therapeutic potential in promoting thyroid cell regeneration, regulating thyroid function, suppressing thyroiditis, and combating thyroid cancer. However, a significant limitation of these studies is the primary reliance on marker expression, while the functional capacity of these differentiated cells to secrete THs remains less extensively validated. This is a crucial area for future research.
| Mechanisms of Action: Immunomodulation and Microenvironment Regulation | ▴Top |
Autoimmune disorders encompass a range of chronic diseases that are typically categorized as organ-specific or systemic [16-18]. These conditions primarily result from immune system dysfunction, in which immune responses are mistakenly directed against the body’s cells and tissues [19, 20]. Stem cell therapy has garnered significant attention as a potential treatment for various diseases, including autoimmune disorders. MSCs are particularly recognized for their immunomodulatory properties, which enable them to restore immune balance in allergic individuals and improve outcomes in tissue and immune-mediated conditions.
MSCs exert both local and systemic immunomodulatory effects. Locally within the thyroid, they reduce lymphocytic infiltration and calm the inflammatory microenvironment. Systemically, they can rebalance immune cell populations by increasing regulatory T cells (Tregs) and decreasing T-helper 17 (Th17) cells in the periphery. MSCs modulate autoimmune responses by secreting paracrine factors such as tumor growth factor-β1 (TGF-β1), interleukin-10 (IL-10), and prostaglandin E2 (PGE2). In models (in vivo) of Hashimoto’s thyroiditis (HT), MSC infusion reduces Th17 cell populations and increases Treg levels, which is associated with a decrease in anti-thyroglobulin antibodies [21]. An increasing number of studies indicate that MSCs impact immune cells through the secretion of factors like TGF-β1, IL-10, and PGE2. Furthermore, MSCs demonstrate unique multi-differentiation capabilities following transplantation [22-24]. The MSCs demonstrate a fibroblast-like spindle morphology. They can be sourced from various tissues, including the umbilical cord, Wharton’s jelly, adipose tissue, bone marrow, dental tissues, and menstrual fluid [25, 26]. In 1966, Friedenstein et al first characterized MSCs as bone-forming cells within bone marrow. However, they are called MSCs due to their pluripotent capabilities as adult stem cells [27]. In vitro studies at the single-cell level have shown that MSCs can differentiate into various cell types, including endothelial cells, cardiomyocytes, and connective tissues such as cartilage and bone [28-30]. MSCs demonstrate immunosuppressive properties mainly through their paracrine effects and interactions with various immune cells. They show low expression of human leukocyte antigen (HLA) class I and rarely express HLA class II. Furthermore, MSCs lack co-stimulatory molecules such as CD40, CD40L, CD80, and CD86, which helps them evade recognition by T cells [31-33]. Furthermore, MSCs have been shown to modulate their local microenvironment, facilitate cellular communication, and release multiple factors that contribute to tissue injury regeneration [34-38]. In a recent preliminary study, individuals with type 1 diabetes mellitus (T1DM) were treated with allogeneic adipose tissue-derived stromal/stem cells (ASCs) at a dose of 1 × 106 cells/kg, along with cholecalciferol 2,000 IU/day for 6 months. Their outcomes were compared to those of a control group [39]. The findings revealed a significant improvement in glycated hemoglobin (HbA1c) levels, with no substantial increase in insulin dosage per kilogram, suggesting a potential upregulation of basal insulin release, as reported by the patients. In summary, ASC therapy is considered safe, with only minimal or transient adverse events. Carlsson et al demonstrated that autologous MSC therapy is both effective and safe for halting the progression of T1DM and for preserving or restoring pancreatic β-cell function in patients with newly diagnosed T1DM [40]. The study reported significant improvements in HbA1c levels and C-peptide concentrations during the follow-up period, with no adverse events observed. These findings suggest that Wharton’s jelly-derived mesenchymal stem cells (WJ-MSCs) infusion is a safe and viable treatment for T1DM. Furthermore, MSCs can benefit rheumatoid arthritis (RA) by inhibiting Th17 cells, reducing inflammatory cytokines, and promoting the upregulation of Treg cells [41].
Furthermore, the low tumorigenicity and minimal immunogenicity of MSCs make them a promising candidate for clinical applications in treating a wide range of diseases and regenerative therapies. MSCs can differentiate from mesodermal origins into various cell types for tissue repair and possess a unique immunomodulatory function. This function involves regulating the release of various anti-inflammatory factors at the immunological level through indirect interactions with soluble cytokines. As a result, MSCs play a crucial role in promoting immune tolerance and maintaining immune homeostasis within the body. It is important to critically note that these compelling immunomodulatory effects are predominantly observed in rodent models of autoimmune thyroiditis, and their translation to human patients necessitates validation through rigorous clinical trials
| Application in Thyroid Nodules and Cancer | ▴Top |
Stem cells are a group of undifferentiated cells with the intrinsic ability to self-renew and, under specific conditions, differentiate into various cell types that form different tissues and organs, therefore aiding in tissue repair. Recent advances have been made in utilizing MSCs and hematopoietic stem cells, which have been induced into thyroid precursor cells, to potentially treat thyroid diseases (Table 1) [10, 21, 42-47]. The development of thyroid cancer in patients is often initially indicated by the presence of a thyroid nodule. Recent progress in stem cell technology has deepened our understanding of the biological and molecular mechanisms driving disease processes. The origin of stem cells and their distinct roles in thyroid cancer remain key areas of ongoing academic research.
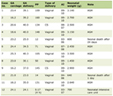 Click to view | Table 1. Summary of Preclinical Studies on Stem Cell Therapy for Thyroid Disorders |
To the best of our knowledge, the exact mechanisms by which MSCs adhere to tumor cells remain unclear. It is suggested that TGF-β1 may play a role in recruiting MSCs to the breast cancer tumor microenvironment. Using neutralizing antibodies against TGF-β1 has been shown to reduce MSCs migration significantly. Moreover, BM-MSCs can be classified as pro-inflammatory or anti-inflammatory, depending on the nature of the surrounding microenvironment. In the cancer microenvironment, these cells differentiate into tumor-like phenotypes. MSCs show paradoxical roles: TGF-β1 secretion may promote anaplastic thyroid cancer invasion [42], while engineered MSC-EVs delivering miR-146b inhibit tumor growth via BRAF/NF-κB targeting [48].
This raises the question: how can BM-MSCs be effectively used in cancer treatment? Given their natural ability to home to tumor sites, bone marrow MSCs are being explored as vehicles for delivering specific therapeutic genes in anti-cancer strategies. The interleukin-4 receptor (IL-4R) binding peptide, IL4RPep-1, can act as a targeting moiety for extracellular vesicles (EVs) in the treatment of mesenchymal thyroid tumors that express the IL-4R [49]. Veschi et al used human embryonic stem cells (hESCs) as a model to study their ability to self-renew and differentiate into mature cell types. By inducing the differentiation of hESCs into thyroid epithelial cells and performing genetic editing with the CRISPR-Cas9 system, they demonstrated that the hierarchical organization of stem cells plays a crucial role in slowing the aging process, aiding in injury recovery, and ultimately protecting cells from the accumulated damage that can lead to cancer [50].
Furthermore, the clinical translation of our findings must be contextualized within existing diagnostic frameworks, such as the Bethesda System for Reporting Thyroid Cytopathology (TBSRTC), which is pivotal for risk stratification and management of thyroid nodules. According to the literature, approximately 70% of all fine-needle aspirations (FNAs) are categorized as Bethesda II [51].While conventional TBSRTC estimates the risk of malignancy (ROM) for Bethesda II (benign) nodules at 1.53% [52] and III (atypia of undetermined significance/follicular lesion of undetermined significance (AUS/FLUS)) at 15%, and the most common histological subtype is the follicular variant of papillary thyroid carcinoma [53]. These data collectively emphasize that the integration of molecular profiling and refined cytopathological subtyping can significantly optimize patient management, potentially reducing unnecessary surgeries for benign nodules while ensuring timely intervention for malignant ones. In the future, our proposed MSC-based therapies could be integrated into such a nuanced diagnostic-therapeutic algorithm, particularly for patients with indeterminate nodules who opt against immediate diagnostic surgery.
Looking forward, the therapeutic potential of MSC-EVs could be enhanced by engineering them to specifically target cancer stem cells (CSCs), a key population driving recurrence and therapy resistance. Concurrently, critical safety strategies must be employed, such as pre-screening MSC donors for oncogenic miRNAs and utilizing advanced EV purification methods to ensure the exclusion of potentially harmful cargo.
| Application in Autoimmune Thyroid Disorders | ▴Top |
Stem cell therapy for thyroiditis
Autoimmune thyroid diseases (AITDs) typically target normally functioning follicular cells, involving numerous cytokines and immune cells [54]. This pathological process often results in hypothyroidism. HT is a key AITD, marked by lymphocytic infiltration, fibrotic changes, and parenchymal atrophy [55]. Recent research suggests that MSCs derived from human fetal umbilical cord tissue hold therapeutic potential for treating Hashimoto’s disease in a rat model, where thyroid lesions and lymphoid infiltration are relatively uncommon. The primary mechanism involves the regulation of Th17/Treg cellular homeostasis [56]. Cao et al conducted a study using 24 female rats with HT to develop a disease model, evaluating the effect of stem cell transplantation on Th17/Treg cellular interactions. In a thyroglobulin-induced experimental autoimmune thyroiditis (EAT) rat model, it was found that stem cell transplantation significantly reduced thyroid lesions compared to the HT model rats. The study demonstrated improved thyroid tissue integrity and a significant reduction in lymphoid infiltration in the thyroid gland, as observed in the EAT model [56]. In rats that received four tail vein injections of either pharmacological agents or human umbilical cord mesenchymal stem cells (hUCMSCs), the hUCMSC treatment significantly decreased inflammation, serum thyroid antibody levels, and TH concentrations. hUCMSC therapy reduced serum thyroid antibody levels, alleviated thyroid inflammation, and balanced CD4+ T cells, highlighting its potential as an effective treatment for autoimmune thyroiditis [57].
Choi et al [58] used both human adipose tissue-derived mesenchymal stem cells (hATMSCs) and genetically engineered hATMSCs expressing mouse cytotoxic T-lymphocyte-associated antigen-4 immunoglobulin (CTLA4Ig) to treat murine models of autoimmune thyroiditis. Both hATMSCs and genetically modified hATMSCs effectively reduced pro-inflammatory cytokine production and restored the Th1/Th2 cell balance in their study. The therapeutic efficacy of adipose tissue-derived mesenchymal stem cells (ATMSCs) from various mouse species was consistent, regardless of whether homograft or allograft transplantation was used [59]. The intercellular adhesion molecule-1 (ICAM-1) plays a role in the homing of MSCs after intravenous administration and influences the release of immune cytokines once MSCs have homed. In a rat model of HT, MSCs reduce thyroid lesions by modulating lymphatic infiltration through regulating Th17/Treg cell interactions [60]. Graves’ disease (GD), a form of AITD, often leads to Graves’ ophthalmopathy. Research suggests that human placental MSCs can suppress orbital fibroblast activation and reduce adipose tissue formation in the orbital region [61, 62].
Stem cell therapy for hyperthyroidism or hypothyroidism
Hypothyroidism is a common endocrine disorder affecting roughly 1-5% of the global population. TH deficiency can result from insufficient synthesis or secretion caused by factors such as external irradiation, genetic defects, congenital hypothyroidism, or treatments like thyroid ablation for hyperthyroidism or thyroid cancer. Clinically, this condition is marked by TH deficiency [6, 63, 64]. In clinical practice, thyroxine tablets are commonly used for replacement therapy; however, long-term use of this medication is associated with several adverse reactions. Recent studies suggest that stem cell therapy could offer an effective therapeutic alternative for managing thyroid disorders, particularly hypothyroidism. Stem cells are multipotent cells with significant differentiation potential, allowing them to be induced into thyroid or thyroid follicular cells or transplanted to treat thyroid conditions like hypothyroidism. Recent advancements in human cell-based models for biological research and disease modeling have been made. Human ESC programs have successfully generated various human organoids, including those of the brain, intestine, stomach, liver, kidney, lung, endometrium, prostate, pancreas, and retina [65, 66]. Organoids derived from ESCs can replicate thyroid gland development and produce THs both in vitro and after transplantation into thyroid-ablated mice. These human ESC-derived thyroid organoids effectively restored plasma TH levels in hypothyroid mice. This demonstrates that the organoids achieved not just structural differentiation but also the essential endocrine function of hormone secretion. In a study, human adipose-derived mesenchymal stem cells (hAMSCs) were administered via tail vein injection into aged female mice with subclinical hypothyroidism (SCH). The results showed that hAMSCs transplantation in AR-SCH mice led to apoptosis of initial thyroid follicular cells and significant improvements in thyroid function, lipid profiles, and cardiac function indices. This suggests that hAMSCs could be a safe and effective treatment for senescent skin changes, potentially reducing complications through immunomodulation and apoptosis inhibition [67]. Moreover, MSC cultures have proven effective in promoting regeneration and healing at fracture sites in the context of hypothyroidism. MSCs retain their multidirectional differentiation potential even after multiple passages, reinforcing their potential for regulating hypothyroidism through MSC therapy [68]. Similarly, studies have shown that bone marrow MSC-derived conditioned medium, in combination with low-level laser therapy, improves fracture regeneration and accelerates bone healing in hypothyroid rats [69].
Stem cells play a crucial role in the treatment of hyperthyroidism by offering potential regenerative therapies that address the root causes of the condition. Hyperthyroidism, commonly known as an overactive thyroid, is characterized by the excessive production and release of THs from the thyroid gland. This overproduction accelerates metabolism and increases sympathetic nervous system activity, leading to symptoms such as palpitations, sweating, increased appetite, frequent bowel movements, and weight loss. Many patients also experience ophthalmic symptoms, such as exophthalmos, eyelid swelling, and vision impairment. The causes of hyperthyroidism include conditions like diffuse toxic goiter (GD), nodular goiter, and autonomously functioning thyroid adenomas [70]. GD, an autoimmune disorder affecting the thyroid gland, accounts for over 80% of hyperthyroidism cases. The exact cause of GD remains largely unknown, though it may be linked to factors such as fever, sleep deprivation, and stress. While current conventional treatments can effectively manage disease progression and alleviate symptoms, individuals with abnormal or refractory thyroid function continue to experience discomfort and require ongoing medication. Graves’ ophthalmoplegia (GO) is a potentially vision-threatening complication of GD, characterized by fibrosis of the extraocular muscles, upper eyelid retraction, and proptosis due to the expansion of orbital adipose tissue [71-74].
The therapeutic strategy for managing hyperthyroidism through stem cells is based on leveraging their ability to differentiate into thyroid follicular epithelial cells, adrenal epithelial cells, adrenergic cells, and other functional cells within the endocrine system. This approach seeks to replace damaged or diseased cells, thus restoring the normal secretion of thyroid, adrenal, and pituitary hormones. As a result, this restoration helps to re-establish the complex regulatory interactions between the endocrine, nervous, and immune systems, ultimately supporting the body’s homeostasis and normal functioning [75]. Replacement therapy involves differentiating human embryonic, induced pluripotent, or MSCs into functional thyroid follicular cells in vitro, followed by transplantation into the body. Our previous study suggests that the dual phenotype observed may result from a heterogeneous cell population, partly attributed to the expression of the MSC marker CD90. Between 45% and 70% of the cells show low levels of CD34 protein expression, with significantly reduced expression in GO cells. In GD affecting the fovea, fibroblasts display osteogenic activity, as evidenced by calcium deposition and the expression of osteocalcin (bone gamma-carboxyglutamic acid-containing protein (BGLAP)) and bone connexin (secreted protein acidic and rich in cysteine (SPARC)). They also demonstrate chondrogenic features, indicated by glycosaminoglycan production and SOX9 and aggrecan protein expression. These fibroblasts show post-differentiation myogenesis through alpha-smooth muscle actin expression and neurogenic activity via beta-III tubulin expression, suggesting that orbital fibroblasts qualify as MSCs. This subpopulation may be increased in GO and could contribute to the disease’s complex differentiation phenotype [76]. In 2011, Zhang et al [76] investigated the in vitro differentiation of human BM-MSCs into thyroid follicular cells under controlled conditions. Their study found that by the third day of induced culture, BM-MSCs underwent morphological changes, transitioning from an elongated, pike-like shape to a more elliptical, round, or triangular form. By the eighth day of differentiation, the formation of walled, flattened embryoid bodies was observed.
Furthermore, the expression of the TSHr gene was noted on day 7, while the TTF-1 gene expression appeared on day 9 of differentiation. These findings suggest that BM-MSCs possess the potential to differentiate into thyroid follicular cells in vitro, offering promising seed cells for tissue engineering research aimed at treating thyroid disorders [76]. In 2018, da Silva et al [77] conducted research in Brazil to examine the effects of MSC therapy on thyroid function and ROS production in diabetic rats. The researchers focused on the thyroid cell line PCCL3 in rats exposed to high glucose levels. The study involved administering either MSCs or secreted EVs over 4 weeks, following an initial 4-week treatment phase in a passaged diabetic rat model.
The findings indicated that treatment with either MSCs or MSC-EV effectively reversed the increased production of thyroid H2O2 in diabetic rat PCCL3 cells, which was linked to a significant upregulation of DuOx1 expression levels [78, 79]. MSC treatment normalized thyroid ROS production, but serum TH levels remained low, and serum TSH concentrations were elevated. MSC treatment did not restore thyroid peroxidase (TPO) levels in the model rats. The research underscores the importance of preclinical and clinical studies on MSCs, exosomes, and related components in treating thyroid dysfunction [80, 81]. Therefore, the researcher concluded that MSC treatment effectively reversed the elevated production of thyroid H2O2 in diabetic animals and PCCL3 cells exposed to high glucose levels. This effect is likely mediated by the paracrine action of MSC-derived EVs in co-culture [81-83].
The research emphasizes the critical need for preclinical and clinical studies on MSCs, exosomes, and related components to explore their potential in treating thyroid dysfunction [84]. In 2017, Sheng et al reviewed the progress in differentiating induced pluripotent stem cells into thyroid follicular cells [84]. The study provided a comprehensive analysis and future perspectives on identifying functionally differentiated cells while addressing safety concerns and various challenges associated with their clinical translational applications.
The results of stem cell therapy for hyperthyroidism are generally significant [85]. At the same time, thyroid function gradually returns to normal, substantially improving overall physiological health. Importantly, stem cell therapy shows a higher safety profile and greater efficacy than traditional treatment methods.
While MSC-based therapies present a promising alternative to traditional thyroid treatments by avoiding the adverse effects of anti-thyroid drugs and radioactive iodine, their clinical application faces three major challenges. First, the risk of tumorigenicity associated with MSC-derived EVs remains a significant concern. Preclinical studies suggest that EVs may transfer oncogenic microRNAs (e.g., miR-155) to thyroid cancer cells, potentially promoting tumor progression through the activation of the PI3K/Akt pathway [43]. Secondly, the heterogeneity of MSC sources such as bone marrow, adipose tissue, or umbilical cord results in inconsistent therapeutic outcomes. For instance, adipose-derived MSCs show higher efficiency in thyrocyte differentiation compared to bone marrow-derived populations, yet there is a lack of standardized protocols for large-scale production [86]. Thirdly, regulatory frameworks have not kept pace with technological advancements. Current regulatory frameworks, including those from the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA), do not fully address the complexity of MSC-EV characterization for advanced therapy medicinal products (ATMPs) [87].
To address these challenges, future research should focus on biomarker-driven safety profiling to identify high-risk MSC subpopulations and explore CRISPR-engineered EVs for the targeted delivery of anti-inflammatory miRNAs or thyrocyte-specific growth factors. Similarly, ethical frameworks should shift from relying on fetal tissue-derived MSCs to using induced pluripotent stem cell (iPSC)-derived alternatives, necessitating rigorous genomic stability testing. Collaborative efforts between biologists, clinicians, and regulators are crucial to standardizing MSC characterization, validating long-term safety through multicenter trials, and developing scalable manufacturing processes. Only through such interdisciplinary collaboration can MSC therapies transition from experimental potential to clinically viable solutions for thyroid disorders.
Therapeutic outcomes may not meet expectations for all patients. Moreover, the high cost of stem cell therapy may place a considerable financial strain on patients. However, with continuous technological advancements and in-depth research, there is a reasonable expectation that the full potential of stem cell therapy for treating endocrine disorders, such as hyperthyroidism, will be better realized in the future.
| Clinical Translation Challenges and Future Directions | ▴Top |
Several translational challenges temper the therapeutic potential of MSCs in treating thyroid disorders. First, the tumorigenicity risks linked to MSC-derived EVs require thorough safety validation. Research suggests that EVs may transfer oncogenic miRNAs, such as miR-155, within thyroid cancer microenvironments, potentially accelerating tumor progression [43]. To mitigate potential risks, such as oncogenic miRNA transfer, practical strategies include the pre-screening of MSC donors and the purification of EVs products. Furthermore, future therapies could be enhanced by engineering these EVs to specifically target therapy-resistant CSCs. Second, the heterogeneity in MSC sources stemming from bone marrow, adipose tissue, or umbilical cord results in inconsistent therapeutic outcomes, highlighting the need for standardized differentiation protocols [47]. Third, regulatory hurdles hinder clinical adoption, as current regulatory frameworks, including those from the US FDA and the EMA, do not fully address the complexity of MSC-EV characterization for ATMPs [88]. To address these challenges, future research should focus on biomarker-driven approaches. Multi-omics profiling of patient-specific MSC populations could help identify tumorigenicity and immunomodulatory efficacy predictive markers. Furthermore, CRISPR-engineered EVs, loaded with thyroid-specific miRNAs (e.g., miR-146b) or anti-inflammatory cytokines (e.g., IL-10), could improve therapeutic precision while reducing off-target effects. At the same time, ethical frameworks should shift away from reliance on fetal MSC sources and promote iPSC-derived alternatives, which would require rigorous genomic stability evaluations. It is important to note that the shift to iPSCs is not without risk, as the reprogramming process can induce genomic instability and mutations, requiring thorough genetic and functional validation of any clinical-grade iPSC line.
Finally, successfully translating MSC-based therapies from research to clinical practice requires collaborative innovation. Interdisciplinary collaboration among biologists, clinicians, and regulators is crucial to developing standardized protocols, ensuring long-term safety, and addressing ethical concerns. By overcoming these obstacles, MSC therapies have the potential to move from theoretical promise to clinically validated treatments for thyroid disorders.
THs play a crucial role in various physiological functions, and hypothyroidism can lead to numerous health complications. Research suggests that remaining thyroid tissue cannot regenerate and restore normal function after surgical intervention due to the slow conversion rate of thyroid glands and the possible involvement of resident stem cells in adult thyroid tissue regeneration. Thyroid organ transplantation offers a promising approach to restoring thyroid function. Researchers have successfully derived thyroid organs from various sources, such as ESCs, iPSCs, and fetal and adult thyroid tissues, demonstrating their functionality in both in vitro and in vivo animal models. iPSCs, created to mimic ESCs, and MSCs, which have broader applications in life sciences, are pivotal in reducing lymphocytic infiltration in autoimmune thyroiditis and limiting epithelial cell proliferation in orbital tissues associated with GD. Furthermore, stem cells within the thyroid gland act as precursors to mature thyroid follicular cells and are believed to play a role in renewing senescent cells and maintaining thyroxine homeostasis. CSCs are often considered the primary drivers of carcinogenesis, making their precise identification and inhibition essential for reversing the carcinogenic process.
The pluripotent capabilities of stem cells offer numerous research and therapeutic intervention opportunities. Future studies are essential to deepen our understanding of the cellular mechanisms involved in thyroid regeneration and to refine protocols for the differentiation and maturation of human thyroid tissues. These investigations provide valuable insights into potential treatments for thyroid disorders and lay a solid scientific foundation for progress in regenerative medicine. Ongoing research into the differentiation of stem cells into thyroid-like cells may pave the way for innovative treatments for thyroid diseases.
Although MSCs offer a transformative approach to managing thyroid disorders, their clinical application depends on addressing three key challenges: 1) Standardizing cell products; 2) Ensuring long-term safety; and 3) Balancing innovation with ethical considerations. Achieving success in the future will require close collaboration between biologists, clinicians, and regulators.
Besides these challenges, our study has several limitations. First, as a comprehensive review, the conclusions drawn are synthesized from existing preclinical (primarily animal studies) and limited early-stage clinical trials. The therapeutic efficacy and safety profiles of MSCs and MSC-EVs documented herein require further validation through large-scale, randomized controlled clinical trials in human patients. Second, substantial heterogeneity in MSC sources, isolation protocols, expansion conditions, and administration doses across the cited studies makes it challenging to directly compare results or establish a standardized therapeutic regimen. Finally, our discussion on the potential of MSCs in treating thyroid disorders is primarily based on mechanistic insights and efficacy data from model systems; the specific impact of these therapies on the diagnostic and risk stratification landscape of thyroid nodules, particularly in the context of challenging Bethesda categories, is yet to be explored and represents an important avenue for future research.
The clinical translation of MSC-based therapies for thyroid disorders necessitates a focused strategy. Future priorities must include: 1) Functional validation of hormone secretion in vivo; 2) Standardization of manufacturing and safety protocols; 3) Initiation of pilot clinical trials; and 4) Early engagement with regulatory agencies (e.g., FDA, EMA). Addressing these steps is crucial to harnessing the full therapeutic potential of MSCs.
Acknowledgments
Yan Fen Liu and Xue Yong Lou acknowledge support from the Medical Health Science and Technology Project of Zhejiang Provincial Health Commission (2021KY1178).
Financial Disclosure
This work was supported by the Medical Health Science and Technology Project of Zhejiang Provincial Health Commission (2021KY1178).
Conflict of Interest
None to declare.
Author Contributions
Yan Fen Liu conducted a literature review, developed the concept for the paper, and authored the manuscript. Xue Yong Lou contributed to the discussion and gave final approval of the version to be published.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Beynon ME, Pinneri K. An overview of the thyroid gland and thyroid-related deaths for the forensic pathologist. Acad Forensic Pathol. 2016;6(2):217-236.
doi pubmed - Mulita F, Anjum F. Thyroid adenoma. In: StatPearls. Treasure Island (FL) ineligible companies. 2025.
pubmed - Piticchio T, Frasca F, Malandrino P, Trimboli P, Carrubba N, Tumminia A, Vinciguerra F, et al. Effect of gluten-free diet on autoimmune thyroiditis progression in patients with no symptoms or histology of celiac disease: a meta-analysis. Front Endocrinol (Lausanne). 2023;14:1200372.
doi pubmed - Uppal N, Collins R, James B. Thyroid nodules: Global, economic, and personal burdens. Front Endocrinol (Lausanne). 2023;14:1113977.
doi pubmed - Seib CD, Sosa JA. Evolving understanding of the epidemiology of thyroid cancer. Endocrinol Metab Clin North Am. 2019;48(1):23-35.
doi pubmed - Taylor PN, Albrecht D, Scholz A, Gutierrez-Buey G, Lazarus JH, Dayan CM, Okosieme OE. Global epidemiology of hyperthyroidism and hypothyroidism. Nat Rev Endocrinol. 2018;14(5):301-316.
doi pubmed - Farini A, Sitzia C, Erratico S, Meregalli M, Torrente Y. Clinical applications of mesenchymal stem cells in chronic diseases. Stem Cells Int. 2014;2014:306573.
doi pubmed - Lener T, Gimona M, Aigner L, Borger V, Buzas E, Camussi G, Chaput N, et al. Applying extracellular vesicles based therapeutics in clinical trials - an ISEV position paper. J Extracell Vesicles. 2015;4:30087.
doi pubmed - Simhadri VR, Andersen JF, Calvo E, Choi SC, Coligan JE, Borrego F. Human CD300a binds to phosphatidylethanolamine and phosphatidylserine, and modulates the phagocytosis of dead cells. Blood. 2012;119(12):2799-2809.
doi pubmed - Mafikandi V, Roodbari NH, Nabiuni M, Yaghmaei A. Effects of maternal thyroid hormone deficiency on differentiation of mesenchymal stem cells in CSF-exposed neonatal Wistar rats. Acta Neurobiol Exp (Wars). 2019;79(3):270-275.
pubmed - Onyshchenko MI, Panyutin IG, Panyutin IV, Neumann RD. Stimulation of cultured h9 human embryonic stem cells with thyroid stimulating hormone does not lead to formation of thyroid-like cells. Stem Cells Int. 2012;2012:634914.
doi pubmed - Okamoto M, Hayase S, Miyakoshi M, Murata T, Kimura S. Stem cell antigen 1-positive mesenchymal cells are the origin of follicular cells during thyroid regeneration. PLoS One. 2013;8(11):e80801.
doi pubmed - Johansson E, Andersson L, Ornros J, Carlsson T, Ingeson-Carlsson C, Liang S, Dahlberg J, et al. Revising the embryonic origin of thyroid C cells in mice and humans. Development. 2015;142(20):3519-3528.
doi pubmed - Wang Y, Tian M, Wang F, Heng BC, Zhou J, Cai Z, Liu H. Understanding the immunological mechanisms of mesenchymal stem cells in allogeneic transplantation: from the aspect of major histocompatibility complex class I. Stem Cells Dev. 2019;28(17):1141-1150.
doi pubmed - Ryu JS, Jeong EJ, Kim JY, Park SJ, Ju WS, Kim CH, Kim JS, et al. Application of mesenchymal stem cells in inflammatory and fibrotic diseases. Int J Mol Sci. 2020;21(21).
doi pubmed - Fugger L, Jensen LT, Rossjohn J. Challenges, Progress, and Prospects of Developing Therapies to Treat Autoimmune Diseases. Cell. 2020;181(1):63-80.
doi pubmed - Ghorbani F, Abbaszadeh H, Mehdizadeh A, Ebrahimi-Warkiani M, Rashidi MR, Yousefi M. Biosensors and nanobiosensors for rapid detection of autoimmune diseases: a review. Mikrochim Acta. 2019;186(12):838.
doi pubmed - Forbes GM, Sturm MJ, Leong RW, Sparrow MP, Segarajasingam D, Cummins AG, Phillips M, et al. A phase 2 study of allogeneic mesenchymal stromal cells for luminal Crohn's disease refractory to biologic therapy. Clin Gastroenterol Hepatol. 2014;12(1):64-71.
doi pubmed - Zhang X, Zambrano A, Lin ZT, Xing Y, Rippy J, Wu T. Immunosensors for biomarker detection in autoimmune diseases. Arch Immunol Ther Exp (Warsz). 2017;65(2):111-121.
doi pubmed - Sinha AA, Lopez MT, McDevitt HO. Autoimmune diseases: the failure of self tolerance. Science. 1990;248(4961):1380-1388.
doi pubmed - Shimoyama T, Miyoshi M, Nehira T, Motojima A, Oikawa T, Laurence O, Igarashi Y. Two new secondary metabolites from a fungus of the genus Robillarda. J Antibiot (Tokyo). 2018;71(4):432-437.
doi pubmed - Rezabakhsh A, Sokullu E, Rahbarghazi R. Applications, challenges and prospects of mesenchymal stem cell exosomes in regenerative medicine. Stem Cell Res Ther. 2021;12(1):521.
doi pubmed - Zakrzewski W, Dobrzynski M, Szymonowicz M, Rybak Z. Stem cells: past, present, and future. Stem Cell Res Ther. 2019;10(1):68.
doi pubmed - Ullah I, Subbarao RB, Rho GJ. Human mesenchymal stem cells - current trends and future prospective. Biosci Rep. 2015;35(2).
doi pubmed - Abbaszadeh H, Ghorbani F, Derakhshani M, Movassaghpour AA, Yousefi M, Talebi M, Shamsasenjan K. Regenerative potential of Wharton's jelly-derived mesenchymal stem cells: A new horizon of stem cell therapy. J Cell Physiol. 2020;235(12):9230-9240.
doi pubmed - Ding DC, Shyu WC, Lin SZ. Mesenchymal stem cells. Cell Transplant. 2011;20(1):5-14.
doi pubmed - Friedenstein AJ, Piatetzky S, II, Petrakova KV. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966;16(3):381-390.
pubmed - Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105(1):93-98.
doi pubmed - Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143-147.
doi pubmed - Cirnu-Georgian L. [Cytogenetic analysis of guerin T8 ascites tumor after 5 years of serial passage]. Bull Cancer. 1971;58(4):485-494.
pubmed - Wang M, Yuan Q, Xie L. Mesenchymal Stem Cell-Based Immunomodulation: Properties and Clinical Application. Stem Cells Int. 2018;2018:3057624.
doi pubmed - Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25(11):2739-2749.
doi pubmed - Sotiropoulou PA, Perez SA, Gritzapis AD, Baxevanis CN, Papamichail M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells. 2006;24(1):74-85.
doi pubmed - Najar M, Martel-Pelletier J, Pelletier JP, Fahmi H. Mesenchymal stromal cell immunology for efficient and safe treatment of osteoarthritis. Front Cell Dev Biol. 2020;8:567813.
doi pubmed - Abbaszadeh H, Ghorbani F, Derakhshani M, Movassaghpour A, Yousefi M. Human umbilical cord mesenchymal stem cell-derived extracellular vesicles: A novel therapeutic paradigm. J Cell Physiol. 2020;235(2):706-717.
doi pubmed - Kim HK, Lee SG, Lee SW, Oh BJ, Kim JH, Kim JA, Lee G, et al. A subset of paracrine factors as efficient biomarkers for predicting vascular regenerative efficacy of mesenchymal stromal/stem cells. Stem Cells. 2019;37(1):77-88.
doi pubmed - Oh EJ, Lee HW, Kalimuthu S, Kim TJ, Kim HM, Baek SH, Zhu L, et al. In vivo migration of mesenchymal stem cells to burn injury sites and their therapeutic effects in a living mouse model. J Control Release. 2018;279:79-88.
doi pubmed - Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med. 2013;45(11):e54.
doi pubmed - Dantas JR, Araujo DB, Silva KR, Souto DL, de Fatima Carvalho Pereira M, Luiz RR, Dos Santos Mantuano M, et al. Adipose tissue-derived stromal/stem cells + cholecalciferol: a pilot study in recent-onset type 1 diabetes patients. Arch Endocrinol Metab. 2021;65(3):342-351.
doi pubmed - Carlsson PO, Schwarcz E, Korsgren O, Le Blanc K. Preserved beta-cell function in type 1 diabetes by mesenchymal stromal cells. Diabetes. 2015;64(2):587-592.
doi pubmed - Liu H, Li R, Liu T, Yang L, Yin G, Xie Q. Immunomodulatory Effects of Mesenchymal Stem Cells and Mesenchymal Stem Cell-Derived Extracellular Vesicles in Rheumatoid Arthritis. Front Immunol. 2020;11:1912.
doi pubmed - Ajawatanawong P, Yanai H, Smittipat N, Disratthakit A, Yamada N, Miyahara R, Nedsuwan S, et al. A novel Ancestral Beijing sublineage of Mycobacterium tuberculosis suggests the transition site to Modern Beijing sublineages. Sci Rep. 2019;9(1):13718.
doi pubmed - Zhu P, Lister C, Dean C. Cold-induced Arabidopsis FRIGIDA nuclear condensates for FLC repression. Nature. 2021;599(7886):657-661.
doi pubmed - Charalampous T, Kay GL, Richardson H, Aydin A, Baldan R, Jeanes C, Rae D, et al. Nanopore metagenomics enables rapid clinical diagnosis of bacterial lower respiratory infection. Nat Biotechnol. 2019;37(7):783-792.
doi pubmed - Kuda-Wedagedara AN, Wang C, Martin PD, Allen MJ. Aqueous Eu(II)-containing complex with bright yellow luminescence. J Am Chem Soc. 2015;137(15):4960-4963.
doi pubmed - Gamboa-Antinolo FM, Poyato-Galan JM. [Conscientious objection of healthcare professionals]. Gac Sanit. 2021;35(4):358-360.
doi pubmed - Sekulovski S, Trowitzsch S. Transfer RNA processing - from a structural and disease perspective. Biol Chem. 2022;403(8-9):749-763.
doi pubmed - Scientists in a time of COVID-19. Nat Plants. 2020;6(6):589.
doi pubmed - Hammoudi Halat D, Soltani A, Dalli R, Alsarraj L, Malki A. Understanding and fostering mental health and well-being among university faculty: a narrative review. J Clin Med. 2023;12(13).
doi pubmed - Veschi V, Turdo A, Modica C, Verona F, Di Franco S, Gaggianesi M, Tirro E, et al. Recapitulating thyroid cancer histotypes through engineering embryonic stem cells. Nat Commun. 2023;14(1):1351.
doi pubmed - Rossi ED, Adeniran AJ, Faquin WC. Pitfalls in thyroid cytopathology. Surg Pathol Clin. 2019;12(4):865-881.
doi pubmed - Mulita F, Iliopoulos F, Tsilivigkos C, Tchabashvili L, Liolis E, Kaplanis C, Perdikaris I, et al. Cancer rate of Bethesda category II thyroid nodules. Med Glas (Zenica). 2022;19(1).
doi pubmed - Mulita F, Plachouri MK, Liolis E, Vailas M, Panagopoulos K, Maroulis I. Patient outcomes following surgical management of thyroid nodules classified as Bethesda category III (AUS/FLUS). Endokrynol Pol. 2021;72(2):143-144.
doi pubmed - Antonelli A, Ferrari SM, Corrado A, Di Domenicantonio A, Fallahi P. Autoimmune thyroid disorders. Autoimmun Rev. 2015;14(2):174-180.
doi pubmed - Petranovic Ovcaricek P, Gorges R, Giovanella L. Autoimmune thyroid diseases. Semin Nucl Med. 2024;54(2):219-236.
doi pubmed - Cao Y, Jin X, Sun Y, Wen W. Therapeutic effect of mesenchymal stem cell on Hashimoto's thyroiditis in a rat model by modulating Th17/Treg cell balance. Autoimmunity. 2020;53(1):35-45.
doi pubmed - Gao J, Hu J, Li P, Che K, Wang F, Yan S. Human umbilical cord mesenchymal stem cells alleviate the imbalance of CD4(+) T cells via protein tyrosine phosphatase non-receptor type 2/signal transducer and activator of transcription 3 signaling in ameliorating experimental autoimmune thyroiditis in rats. Endocr J. 2022;69(6):613-625.
doi pubmed - Choi SA, Lee JY, Kwon SE, Wang KC, Phi JH, Choi JW, Jin X, et al. Human adipose tissue-derived mesenchymal stem cells target brain tumor-initiating cells. PLoS One. 2015;10(6):e0129292.
doi pubmed - Choi SA, Yun JW, Joo KM, Lee JY, Kwak PA, Lee YE, You JR, et al. Preclinical biosafety evaluation of genetically modified human adipose tissue-derived mesenchymal stem cells for clinical applications to brainstem glioma. Stem Cells Dev. 2016;25(12):897-908.
doi pubmed - Li X, Wang Q, Ding L, Wang YX, Zhao ZD, Mao N, Wu CT, et al. Intercellular adhesion molecule-1 enhances the therapeutic effects of MSCs in a dextran sulfate sodium-induced colitis models by promoting MSCs homing to murine colons and spleens. Stem Cell Res Ther. 2019;10(1):267.
doi pubmed - Gulbins A, Horstmann M, Keitsch S, Soddemann M, Wilker B, Wilson GC, Zeidan R, et al. Potential involvement of the bone marrow in experimental Graves' disease and thyroid eye disease. Front Endocrinol (Lausanne). 2023;14:1252727.
doi pubmed - Shin HA, Park M, Banga JP, Lew H. TGFbeta-treated placenta-derived mesenchymal stem cells selectively promote anti-adipogenesis in thyroid-associated ophthalmopathy. Int J Mol Sci. 2022;23(10).
doi pubmed - Garmendia Madariaga A, Santos Palacios S, Guillen-Grima F, Galofre JC. The incidence and prevalence of thyroid dysfunction in Europe: a meta-analysis. J Clin Endocrinol Metab. 2014;99(3):923-931.
doi pubmed - Vanderpump MP. The epidemiology of thyroid disease. Br Med Bull. 2011;99:39-51.
doi pubmed - Kim J, Koo BK, Knoblich JA. Human organoids: model systems for human biology and medicine. Nat Rev Mol Cell Biol. 2020;21(10):571-584.
doi pubmed - Clevers H. Modeling development and disease with organoids. Cell. 2016;165(7):1586-1597.
doi pubmed - Li C, Rui Q, Dong X, Ning S, Zhou J, Wu H, Jiang C, et al. Human amnion-derived mesenchymal stem cells improve subclinical hypothyroidism by immunocompetence mediating apoptosis inhibition on thyroid cells in aged mice. Cell Tissue Res. 2023;394(2):309-323.
doi pubmed - Sefati N, Norouzian M, Abbaszadeh HA, Abdollahifar MA, Amini A, Bagheri M, Aryan A, et al. Effects of bone marrow mesenchymal stem cells-conditioned medium on tibial partial osteotomy model of fracture healing in hypothyroidism rats. Iran Biomed J. 2018;22(2):90-98.
doi pubmed - Sefati N, Abbaszadeh HA, Fadaei Fathabady F, Abdollahifar MA, Khoramgah MS, Darabi S, Amini A, et al. The combined effects of mesenchymal stem cell conditioned media and low-level laser on stereological and biomechanical parameter in hypothyroidism rat model. J Lasers Med Sci. 2018;9(4):243-248.
doi pubmed - Kravets I. Hyperthyroidism: diagnosis and treatment. Am Fam Physician. 2016;93(5):363-370.
pubmed - Valmsen K, Boeglin WE, Jarving I, Schneider C, Varvas K, Brash AR, Samel N. Structural and functional comparison of 15S- and 15R-specific cyclooxygenases from the coral Plexaura homomalla. Eur J Biochem. 2004;271(17):3533-3538.
doi pubmed - El-Kaissi S, Frauman AG, Wall JR. Thyroid-associated ophthalmopathy: a practical guide to classification, natural history and management. Intern Med J. 2004;34(8):482-491.
doi pubmed - Tallstedt L, Lundell G, Torring O, Wallin G, Ljunggren JG, Blomgren H, Taube A. Occurrence of ophthalmopathy after treatment for Graves' hyperthyroidism. The Thyroid Study Group. N Engl J Med. 1992;326(26):1733-1738.
doi pubmed - Trobe JD. Optic nerve involvement in dysthyroidism. Ophthalmology. 1981;88(6):488-492.
doi pubmed - Tian Z, Yu T, Liu J, Wang T, Higuchi A. Introduction to stem cells. Prog Mol Biol Transl Sci. 2023;199:3-32.
doi pubmed - Zhang Qin. Engineering Research and Clinical Rehabilitation. Chinese Journal of Tissue. Invest Ophthalmol Vis Sci. 2011:15(6):951-954.
- da Silva D, de Freitas ML, Cahil GM, de Sao Jose VS, Neto FM, Cardoso RC, da Costa VMC, et al. Influence of stem cell therapy on thyroid function and reactive oxygen species production in diabetic rats. Horm Metab Res. 2018;50(4):331-339.
doi pubmed - Bai S, Xiong X, Tang B, Ji T, Li X, Qu X, Li W. hsa-miR-199b-3p prevents the epithelial-mesenchymal transition and dysfunction of the renal tubule by regulating E-cadherin through targeting KDM6A in diabetic nephropathy. Oxid Med Cell Longev. 2021;2021:8814163.
doi pubmed - Eapen MS, McAlinden KD, Myers S, Lu W, Sohal SS. microRNAs are key regulators in chronic lung disease: exploring the vital link between disease progression and lung cancer. J Clin Med. 2019;8(11).
doi pubmed - Liu X, Wang R, Li M, Chen G. Pituitary metastasis of lung neuroendocrine carcinoma mimicking pituitary adenoma: case report and literature review. Front Endocrinol (Lausanne). 2021;12:678947.
doi pubmed - Sim K, Mijakoski D, Stoleski S, Del Rio PR, Sammut P, Le TM, Munblit D, et al. Outcomes for clinical trials of food allergy treatments. Ann Allergy Asthma Immunol. 2020;125(5):535-542.
doi pubmed - Kwon YJ, Lee H, Baik SJ, Chang HJ, Lee JW. Comparison of a machine learning method and various equations for estimating low-density lipoprotein cholesterol in korean populations. Front Cardiovasc Med. 2022;9:824574.
doi pubmed - Badhe RV, Adkine D, Godse A. Development of polylactic acid and bovine serum albumin-layered-coated chitosan microneedles using novel bees wax mould. Turk J Pharm Sci. 2021;18(3):367-375.
doi pubmed - Sheng JG, Zhang JQ. Differentiation of cells into thyroid follicular cells. Chinese Journal of Cell and Stem Cells. 2017:7(3):185-188.
- Zheng XL, Yan HM, Xiao L, Han DM, Ding L, Xue M, Zhu L, et al. [Hyperthyroidism after allogeneic hematopoietic stem cell transplantation]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2022;30(4):1244-1247.
doi pubmed - Liu H, Gu R, Huang Q, Liu Y, Liu C, Liao S, Feng W, et al. Isoliensinine suppresses osteoclast formation through NF-kappaB signaling pathways and relieves ovariectomy-induced bone loss. Front Pharmacol. 2022;13:870553.
doi pubmed - von Cube M, Grodd M, Wolkewitz M, Hazard D, Wengenmayer T, Canet E, Lambert J. Harmonizing heterogeneous endpoints in coronavirus disease 2019 trials without loss of information. Crit Care Med. 2021;49(1):e11-e19.
doi pubmed - Glassman CR, Parrish HL, Lee MS, Kuhns MS. Reciprocal TCR-CD3 and CD4 engagement of a nucleating pMHCII stabilizes a functional receptor macrocomplex. Cell Rep. 2018;22(5):1263-1275.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Endocrinology and Metabolism is published by Elmer Press Inc.