| Journal of Endocrinology and Metabolism, ISSN 1923-2861 print, 1923-287X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Endocrinol Metab and Elmer Press Inc |
| Journal website https://jem.elmerpub.com |
Original Article
Volume 15, Number 4, October 2025, pages 137-143
Efficacy of Second Radioactive Iodine Therapy Based on Serum Thyroglobulin for Papillary Thyroid Cancer: A Retrospective Cohort Study
Hee-Sung Songa, Ji Young Leea, b
aDepartment of Nuclear Medicine, Jeju National University Hospital, Jeju National University School of Medicine, Jeju-si, Korea
bCorresponding Author: Ji Young Lee, Department of Nuclear Medicine, Jeju National University Hospital, Jeju National University School of Medicine, Jeju-si 63241, Korea
Manuscript submitted July 8, 2025, accepted August 23, 2025, published online October 6, 2025
Short title: Second RAI Therapy for PTC
doi: https://doi.org/10.14740/jem1545
| Abstract | ▴Top |
Background: Radioactive iodine (RAI) therapy is widely used in differentiated thyroid cancer to eliminate residual cancer cells and to target metastatic lesions; however, this therapy is associated with a recurrence rate of approximately 2%. Repeat RAI therapy may be considered in patients with persistent or recurrent lesions; however, the benefits of second RAI remain unclear. In this retrospective cohort study, we evaluated the clinical impact of second RAI therapy in patients with papillary thyroid cancer (PTC) and identified potential predictors of treatment response and long-term remission.
Methods: Twenty-one patients who underwent a second round of RAI therapy for PTC were enrolled. Clinical and pathological data, including RAI dosage, second pre- and post-RAI therapy thyroglobulin (Tg) levels, post-treatment scan findings, and Tg reduction rates, were retrospectively analyzed. Treatment responses were evaluated based on changes in the second pre- and post-suppressed Tg (on-Tg) levels, and patients were grouped accordingly. Correlation and comparative analyses were performed to identify potential predictors of response and long-term outcomes.
Results: Following second RAI therapy, 11 patients (52.4%) showed a reduction in on-Tg levels, seven showed no reduction, and three were indeterminate. Higher second pre-therapy on-Tg levels, second therapy-stimulated Tg levels, and second post-therapy on-Tg levels were significantly associated with the no-response group (all P < 0.01) and correlated with the on-Tg difference rate. The indication for a second RAI (structural vs. biochemical incomplete response) and a history of reoperation also differed significantly between the groups. However, at the long-term follow-up, no clinical parameters were significantly associated with achieving no evidence of disease status.
Conclusions: Second RAI therapy was more effective in patients with structural incomplete response and in those who underwent reoperation. While second therapy Tg levels seemed to be correlated with short-term response, clinical factors and Tg levels were not predictive of long-term remission, highlighting the importance of individualized treatment planning.
Keywords: Second radioiodine therapy; Papillary thyroid carcinoma; Iodine-131; Thyroglobulin
| Introduction | ▴Top |
Radioactive iodine (RAI) therapy has been widely used to treat differentiated thyroid cancer (DTC) for over 60 years, with the goal of eliminating residual thyroid tissue and cancer cells following total thyroidectomy while targeting metastatic lesions [1, 2]. The reported success rate of residual thyroid removal following initial RAI therapy ranges from 43% to 94% [3-5]. However, even among patients who achieve successful ablation with primary RAI therapy, the average recurrence rate is approximately 2%, with a reported range of 0% to 7% [5].
Repeat RAI therapy may be considered in patients with persistent or recurrent lesions after initial RAI treatment. The 2015 American Thyroid Association (ATA) guidelines suggest that an empirical dose of 100 - 200 mCi, or a dose-directed approach, may be considered for patients with rapidly rising serum thyroglobulin (Tg) or anti-Tg antibody (TgAb) levels with negative anatomical imaging findings. Surgery is preferred for regional lymph node metastases if the metastatic nodes are large or surgically accessible [6], while adjuvant RAI therapy may be performed after surgery when the metastatic location is difficult to locate or in cases with a possibility of radioiodine uptake. However, the benefits of a second round of RAI therapy remain unclear. Several studies have suggested that repeat RAI therapy may be effective in selecting patients, while others have found no clear benefit from second RAI therapy [7-13].
Numerous studies have investigated the effects of second RAI therapy in the context of various thyroid cancer-related factors, including histological type, stage, tumor size, lymph node metastasis, RAI avidity, and molecular markers [11, 12]. Among these, serum Tg measurement is considered an effective method for monitoring patients with thyroid cancer, as Tg is produced exclusively by thyroid follicular cells, and is a sensitive indicator for predicting local recurrence or metastasis following the resection of residual thyroid tissue [14-16].
Although several studies have previously investigated the effects of a second RAI therapy, none have specifically measured changes in Tg levels in individual patients. This study therefore aimed to retrospectively analyze the effects of a second RAI therapy and identify potential factors affecting treatment response and long-term remission.
| Materials and Methods | ▴Top |
Patients
Approval of this study was obtained from the Institutional Review Board (IRB no. JEJUNUH 2025-06-013). The need for informed consent was waived due to the retrospective study design. We retrospectively reviewed the medical records of 428 patients with histopathologically-confirmed classic papillary thyroid carcinoma, who underwent first RAI treatment at our Nuclear Medicine Department between January 2015 and December 2024. Among these, 21 patients further underwent a second RAI therapy because of persistent or recurrent thyroid disease. Data on pathological findings, RAI dosage, pre- and post-therapy Tg levels, post-therapy scan uptake, and Tg reduction rates were obtained from the patient records.
Protocol for second RAI therapy
Patients who received second RAI therapy did so due to elevated Tg levels or newly detected lymph node metastases following the first RAI therapy. All patients underwent ultrasonography (USG) and/or neck computed tomography (CT). Patients with rising Tg/TgAb levels without structural evidence were classified as having a biochemical incomplete response (BIR), whereas those with anatomical evidence of disease, regardless of Tg levels, were classified as having a structural incomplete response (SIR) [6].
All patients followed a strict protocol for prolonged thyroid hormone withdrawal before the second RAI therapy, which included the discontinuation of levothyroxine for 3 weeks and a low-iodine diet for 2 weeks prior to treatment. Pre-therapy suppressed Tg levels (second pre on-Tg) and stimulated Tg levels (second pre sTg) were measured at the time of the second I-131 administration. The radioactivity administered for the second RAI ablation (3,700 - 11,100 MBq) was determined based on the risk classification of the American Joint Committee on Cancer tumor-node-metastasis staging, surgical and histopathological findings, and the patient’s condition.
Assessment of second RAI treatment
Response assessment was performed by evaluating changes in suppressed Tg levels approximately 6 months following the second RAI therapy (second post on-Tg). A decrease in post-therapy on-Tg levels compared to the second pre on-Tg level indicated a treatment response. However, three patients had undetectable Tg levels (< 0.1 ng/mL) and were therefore excluded from the analysis of Tg level response. On long-term follow-up, no evidence of disease (NED) status was defined in accordance with the ATA guidelines as: on-Tg < 1.0 ng/mL, normal USG findings without palpable cervical lymph nodes, and Tg antibody levels ≤ 100 ng/mL [6].
Statistical analysis
Fisher’s exact test or the Mann-Whitney U test was applied to compare variables between the response and no-response groups following the second RAI therapy. Spearman’s rank correlation analysis was conducted to explore the relationships between multiple variables. Analyses were conducted using MedCalc for Windows, version 12.7 (MedCalc Software, Ostend, Belgium), with statistical significance set at P < 0.05.
| Results | ▴Top |
Of the 21 enrolled patients (16 females, five males; median age, 52 years), 11 (52.4%) showed a decrease in post-therapy on-Tg levels, while seven showed no reduction, and three were classified as indeterminate. The median second RAI therapy dose was 5,550 MBq, and the median interval between the first and second RAI treatments was 498 days. All 14 SIR patients had lymph node metastases in the neck based on USG/CT findings, and 13 patients among them (92.9%) underwent reoperation, primarily neck dissection. The average interval from secondary surgery to the second pre on-Tg measurement in the SIR group was 102 days. In the BIR group, the decision for a second RAI therapy was based on a consistent upward trend in Tg levels rather than a single elevated value after the first RAI therapy. The SIR group showed significantly lower second pre on-Tg (10.5 ± 27.1 vs. 116.8 ± 185.5; P = 0.028) and second pre sTg levels (58.5 ± 106.1 vs. 275.6 ± 224.4; P = 0.012) compared to those of the BIR group.
Table 1 presents a comparison of the key clinical factors between the response and no-response groups after the second RAI therapy. In the response group, the median percentage decreased by 54.6% (22.8 - 75.5) between second pre and post on-Tg after second RAI therapy. Interestingly, the reason for second RAI treatment differed significantly between the two groups (P = 0.002). A larger proportion of Tg responders (90.9%) underwent second RAI therapy due to SIR, whereas most non-responders (85.7%) underwent second RAI due to BIR. Reoperation also significantly differed between the groups (P = 0.049).
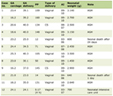 Click to view | Table 1. Differences in Key Clinical Factors Between the Response and No-Response Groups After Second RAI Therapy |
The values of second pre on-Tg and second pre sTg were markedly higher in non-responders (P = 0.008, 0.003), as was the second post on-Tg (P = 0.002). No significant differences were identified in the second dose of RAI therapy or in the presence of uptake on the second iodine scan between the groups. In subgroup analyses based on the indication for a second RAI therapy, no significant variables were associated with therapeutic response within either the BIR or SIR groups.
On the correlation analysis, the on-Tg difference rate demonstrated a statistically significant positive association with several Tg measurements. Specifically, we identified a moderate correlation with the second pre on-Tg (ρ = 0.494, P = 0.039), a strong correlation with the second pre sTg (ρ = 0.685, P = 0.002) and second post on-Tg (ρ = 0.685, P = 0.002). In contrast, no statistically significant correlations were observed between the on-Tg difference rates and other clinical or treatment-related variables (Fig. 1).
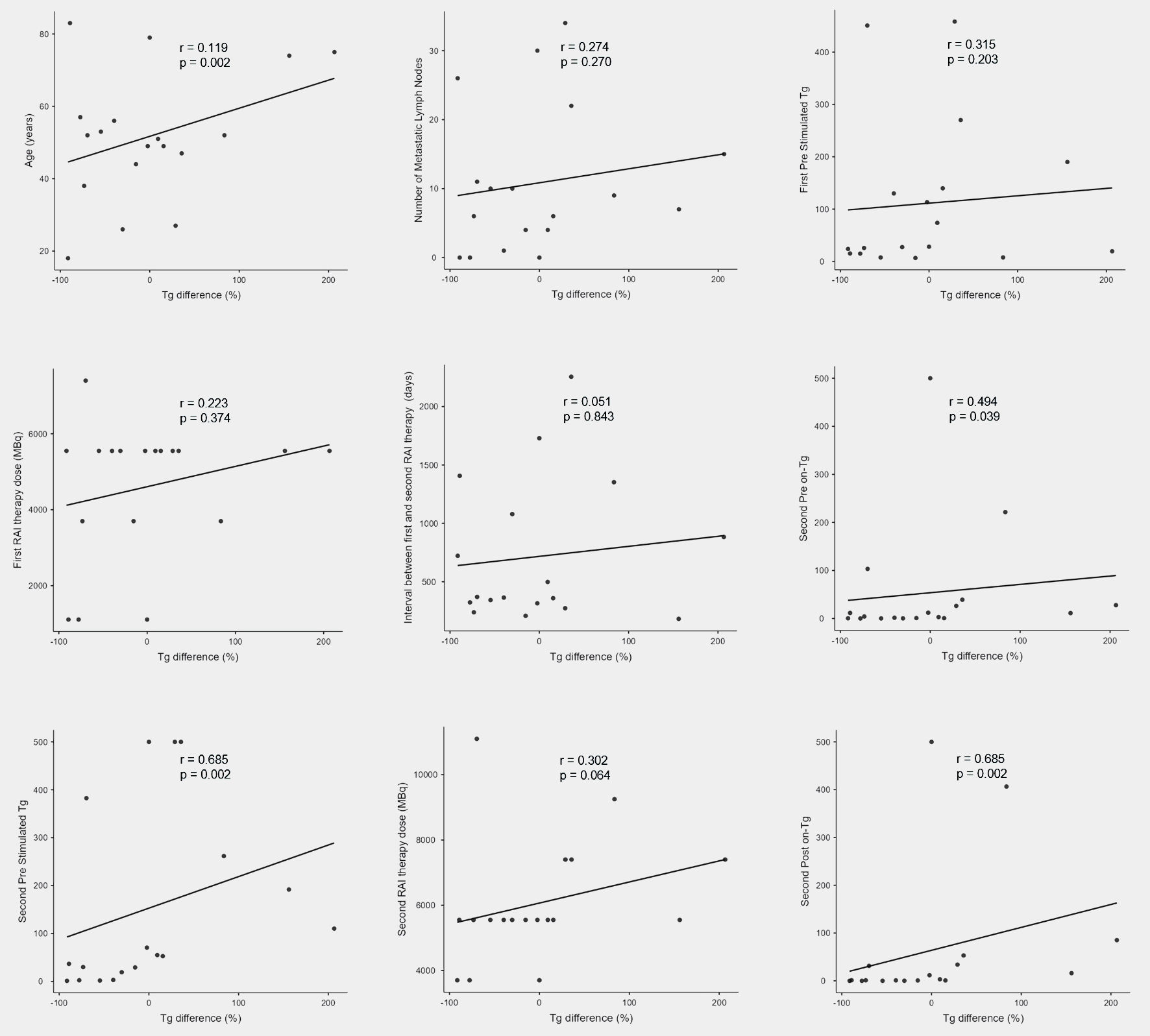 Click for large image | Figure 1. Results of correlation analyses between Tg difference (%) and various clinical parameters. RAI: radioactive iodine; Tg: thyroglobulin. |
At long-term follow-up, 52.4% of patients were in NED. We subsequently compared the clinical parameters between patients with NED and those with incomplete remission to explore potential predictive factors for treatment success, with results showing that age, sex, nodal metastasis, first RAI treatment dose, second RAI treatment dose, and cause of re-RAI treatment were not significantly different between the two groups (Table 2). Although the T3 and T4 stages were more prevalent in both groups, the distribution of disease stages did not significantly differ. Although the median first and second pre sTg levels were higher in the incomplete remission group, the differences were not significant. Furthermore, the range of differences in Tg levels was not significantly different between the two groups.
 Click to view | Table 2. Differences in Key Clinical Factors Between the NED and Incomplete Remission Groups After Second RAI Therapy |
| Discussion | ▴Top |
The results of this study demonstrated that the second pre on-Tg, second pre sTg, and second post on-Tg levels differed significantly between the response and no-response groups after the second RAI therapy. Additionally, the reason for the second RAI therapy (SIR vs. BIR) and the history of reoperation also varied significantly between the groups.
Numerous prior studies have explored the effectiveness of second RAI therapy [8-13, 17, 18]. However, the overall benefits of this approach still remain uncertain. Some of these studies have suggested that repeat RAI therapy may be effective in certain patients. For example, in the study by Cadena-Pineros et al, 48/1,299 patients received secondary RAI therapy after reoperation, of whom 77.1% experienced no further recurrence [8]. In contrast, one study enrolling 45 patients who underwent reoperation for local recurrence identified no clear benefit from secondary RAI therapy [13]. Further, another study involving 53 patients with local recurrence, 61 with elevated Tg levels, and 50 who underwent reoperation suggested that secondary RAI therapy had a limited impact on outcomes in patients with either local recurrence or persistent disease [10]. Therefore, the therapeutic effect of a second RAI treatment remains unclear, and numerous studies have aimed to clarify its role by examining various thyroid cancer-related factors.
Our study is the first to compare individual Tg levels before and 6 months after the second RAI therapy. In our analysis, 52.4 % of patients showed a decrease in on-Tg levels following second RAI treatment. Three patients were excluded from the analysis because of undetectable Tg levels; however, two showed decreased changes in their TgAb levels. These patients were excluded to avoid a potential selection bias, although the percentage of patients showing a response increased. When changes in TgAb levels were included in the analysis, the results were consistent with the primary findings. Specifically, second pre Tg levels and underlying causes remained significantly different between the two groups (data not shown). However, because it was considered methodologically inappropriate to analyze Tg and TgAb differences simultaneously, these three patients were excluded from the treatment-response analysis.
Serum Tg is a well-established biomarker for monitoring patients with DTC, given its exclusive production by thyroid follicular cells [19, 20]. Postoperative Tg levels serve as sensitive indicators for detecting residual disease, recurrence, and metastasis after thyroidectomy and RAI ablation. In this study, we observed that patients with lower Tg levels (second pre on-Tg, second pre sTg, and second post on-Tg) were more likely to respond favorably to a second RAI therapy. These findings reinforce the clinical relevance of Tg monitoring as a predictor of the treatment response. Conversely, Tg elevation during second RAI treatment may indicate a more aggressive or refractory disease course, underscoring the need to consider alternative therapeutic strategies or close surveillance.
The statistically significant correlation between the on-Tg difference rate and the second RAI therapy Tg measurements suggests that the second pre/post on-Tg and second pre sTg levels may serve as valuable indicators of treatment response and tumor burden. In particular, its strong association with the second pre sTg level underscores its potential use as a sensitive biomarker for treatment planning [21, 22]. Although sTg was not consistently measured 6 months post-RAI therapy in all patients, our findings support the utility of serial Tg monitoring, particularly around the second RAI treatment, during disease monitoring and management.
Our study demonstrated that a second RAI therapy was significantly more effective in patients with lymph node involvement. Because most SIR patients underwent secondary surgery, their Tg levels were relatively lower than those in the BIR group. As the second pre Tg levels in the SIR group were measured after surgery, the surgical intervention likely had a major impact. Although prior studies have shown that second RAI therapy is generally ineffective in patients with SIR after surgery, our study demonstrated a 90.9% decrease in Tg levels at 6 months compared to the BIR group [9, 17, 23, 24]. However, long-term follow-up did not reveal a significant difference between the two groups. This outcome may be attributed to the fact that most SIR patients underwent secondary surgery prior to the second RAI therapy, which likely contributed to the more immediate reduction in Tg levels. These findings suggest that while surgery may provide short-term benefits in lowering Tg levels, more comprehensive long-term follow-up is necessary to assess its sustained impact.
This study provides the clinical characteristics and outcomes of patients undergoing a second RAI therapy, particularly in relation to achieving NED status. Although differences were observed in age, sex, and Tg levels, no significant clinical predictors of remission status were identified. These findings suggest a limited prognostic value of traditional biochemical and clinical markers for predicting long-term outcomes. However, most patients remained under follow-up, and only one patient died of thyroid cancer during the study period. No significant clinical predictors of NED could be identified.
This study had several limitations. A major limitation of our study is the small sample size, primarily due to institutional constraints. We also aimed to stratify patients more precisely; however, patients with both BIR and SIR after the first RAI therapy were included due to the limited number of eligible cases, which may have introduced selection bias. Subgroup analyses of the BIR and SIR groups were performed separately; however, no significant predictors were identified, likely owing to the small sample size. Furthermore, the study was conducted at a single institution and primarily evaluated short-term outcomes. The limited sample size reduces statistical power to detect significant associations and increases the margin of error for parameter estimates. To validate the effectiveness and optimal application of a second RAI therapy, future multicenter prospective studies with larger cohorts and longer follow-up are essential. Such studies could also help identify more robust clinical or molecular predictors of treatment response and long-term remission.
Conclusions
Overall, the results of this analysis showed that the response to second RAI therapy in DTC patients is influenced by the pre/post-second RAI treatment Tg levels, cause of therapy, and surgical history. While long-term outcomes, such as NED status, did not correlate with specific clinical predictors, these findings highlight the potential value of individualized treatment planning based on early biochemical and clinical factors.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
The need for informed consent was waived due to the retrospective study design.
Author Contributions
Hee-Sung Song: conceptualization, methodology, formal analysis, investigation, data curation, writing - original draft, and visualization. Ji Young Lee: supervision, project administration, writing - review and editing. All authors read and approved the final manuscript.
Data Availability
The datasets generated and/or analyzed during the current study are not publicly available but are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Bal CS, Kumar A, Pant GS. Radioiodine dose for remnant ablation in differentiated thyroid carcinoma: a randomized clinical trial in 509 patients. J Clin Endocrinol Metab. 2004;89(4):1666-1673.
doi pubmed - Maenpaa HO, Heikkonen J, Vaalavirta L, Tenhunen M, Joensuu H. Low vs. high radioiodine activity to ablate the thyroid after thyroidectomy for cancer: a randomized study. PLoS One. 2008;3(4):e1885.
doi pubmed - Wang C, Diao H, Ren P, Wang X, Wang Y, Zhao W. Efficacy and affecting factors of (131)I thyroid remnant ablation after surgical treatment of differentiated thyroid carcinoma. Front Oncol. 2018;8:640.
doi pubmed - Verburg FA, Lassmann M, Mader U, Luster M, Reiners C, Hanscheid H. The absorbed dose to the blood is a better predictor of ablation success than the administered 131I activity in thyroid cancer patients. Eur J Nucl Med Mol Imaging. 2011;38(4):673-680.
doi pubmed - Klain M, Nappi C, Zampella E, Cantoni V, Green R, Piscopo L, Volpe F, et al. Ablation rate after radioactive iodine therapy in patients with differentiated thyroid cancer at intermediate or high risk of recurrence: a systematic review and a meta-analysis. Eur J Nucl Med Mol Imaging. 2021;48(13):4437-4444.
doi pubmed - Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, et al. 2015 American Thyroid Association Management Guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1-133.
doi pubmed - Bouvet C, Barres B, Kwiatkowski F, Batisse-Lignier M, Chafai El Alaoui M, Kauffmann P, Cachin F, et al. Re-treatment with adjuvant radioactive iodine does not improve recurrence-free survival of patients with differentiated thyroid cancer. Front Endocrinol (Lausanne). 2019;10:671.
doi pubmed - Cadena-Pineros E, Escobar JV, Carreno JA, Rojas JG. Second adjuvant radioiodine therapy after reoperation for locoregionally persistent or recurrent papillary thyroid carcinoma. World J Nucl Med. 2022;21(4):290-295.
doi pubmed - Hirsch D, Gorshtein A, Robenshtok E, Masri-Iraqi H, Akirov A, Duskin Bitan H, Shimon I, et al. Second radioiodine treatment: limited benefit for differentiated thyroid cancer with locoregional persistent disease. J Clin Endocrinol Metab. 2018;103(2):469-476.
doi pubmed - Hung ML, Wu JX, Li N, Livhits MJ, Yeh MW. Association of radioactive iodine administration after reoperation with outcomes among patients with recurrent or persistent papillary thyroid cancer. JAMA Surg. 2018;153(12):1098-1104.
doi pubmed - Prpic M, Kruljac I, Kust D, Kirigin LS, Jukic T, Dabelic N, Bolanca A, et al. Re-ablation I-131 activity does not predict treatment success in low- and intermediate-risk patients with differentiated thyroid carcinoma. Endocrine. 2016;52(3):602-608.
doi pubmed - Wang L, Yun C, Huang F, Xiao J, Ju Y, Cheng F, Zhang W, et al. Preablative stimulated thyroglobulin and thyroglobulin reduction index as decision-making markers for second radioactive iodine therapy in patients with structural incomplete response. Cancer Manag Res. 2021;13:5351-5360.
doi pubmed - Yim JH, Kim WB, Kim EY, Kim WG, Kim TY, Ryu JS, Moon DH, et al. Adjuvant radioactive therapy after reoperation for locoregionally recurrent papillary thyroid cancer in patients who initially underwent total thyroidectomy and high-dose remnant ablation. J Clin Endocrinol Metab. 2011;96(12):3695-3700.
doi pubmed - Pelttari H, Valimaki MJ, Loyttyniemi E, Schalin-Jantti C. Post-ablative serum thyroglobulin is an independent predictor of recurrence in low-risk differentiated thyroid carcinoma: a 16-year follow-up study. Eur J Endocrinol. 2010;163(5):757-763.
doi pubmed - Webb RC, Howard RS, Stojadinovic A, Gaitonde DY, Wallace MK, Ahmed J, Burch HB. The utility of serum thyroglobulin measurement at the time of remnant ablation for predicting disease-free status in patients with differentiated thyroid cancer: a meta-analysis involving 3947 patients. J Clin Endocrinol Metab. 2012;97(8):2754-2763.
doi pubmed - Kendler DB, Vaisman F, Corbo R, Martins R, Vaisman M. Preablation stimulated thyroglobulin is a good predictor of successful ablation in patients with differentiated thyroid cancer. Clin Nucl Med. 2012;37(6):545-549.
doi pubmed - Sicilia Pozo MLN, Pena Pardo FJ, Amo Salas M, Cruz Montijano M, Torres Hernandez J, Padilla Bermejo A, Montalban Mendez C, et al. Second radioiodine treatment in patients with differentiated thyroid carcinoma: Causes and effects. Endocrinol Diabetes Nutr (Engl Ed). 2024;71(1):4-11.
doi pubmed - Xiao C, Xu R, Luo Y, Xu Z, Tang C. Is second (131)I treatment necessary for differentiated thyroid cancer patients and who could not benefit from it? A real-world retrospective study in China. Ann Nucl Med. 2025;39(2):167-175.
doi pubmed - Prpic M, Franceschi M, Romic M, Jukic T, Kusic Z. Thyroglobulin as a tumor marker in differentiated thyroid cancer - clinical considerations. Acta Clin Croat. 2018;57(3):518-527.
doi pubmed - Scharpf J, Tuttle M, Wong R, Ridge D, Smith R, Hartl D, Levine R, et al. Comprehensive management of recurrent thyroid cancer: An American Head and Neck Society consensus statement: AHNS consensus statement. Head Neck. 2016;38(12):1862-1869.
doi pubmed - Barres B, Kelly A, Kwiatkowski F, Batisse-Lignier M, Fouilhoux G, Aubert B, Dutheil F, et al. Stimulated thyroglobulin and thyroglobulin reduction index predict excellent response in differentiated thyroid cancers. J Clin Endocrinol Metab. 2019;104(8):3462-3472.
doi pubmed - Yang X, Liang J, Li TJ, Yang K, Liang DQ, Yu Z, Lin YS. Postoperative stimulated thyroglobulin level and recurrence risk stratification in differentiated thyroid cancer. Chin Med J (Engl). 2015;128(8):1058-1064.
doi pubmed - Gambale C, Prete A, Contartese L, Torregrossa L, Bianchi F, Molinaro E, Materazzi G, et al. Usefulness of second 131I treatment in biochemical persistent differentiated thyroid cancer patients. Eur Thyroid J. 2023;12(6):e230052.
doi pubmed - Vaisman F, Tala H, Grewal R, Tuttle RM. In differentiated thyroid cancer, an incomplete structural response to therapy is associated with significantly worse clinical outcomes than only an incomplete thyroglobulin response. Thyroid. 2011;21(12):1317-1322.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Endocrinology and Metabolism is published by Elmer Press Inc.