Effect of Imeglimin on Microalbuminuria and Serum Vitamin B12 Concentration in Japanese Patients With Type 2 Diabetes
DOI:
https://doi.org/10.14740/jem1537Keywords:
Imeglimin, Microalbuminuria, Vitamin B12, Metformin, Type 2 diabetesAbstract
Background: Imeglimin is a unique oral anti-diabetic agent with extra- and intra-pancreatic actions. Its safety and efficacy were demonstrated by a multicenter phase 3 trial, but its effect on albuminuria has not been fully evaluated. Metformin is the most widely used first-line anti-diabetic agent, but it partially blocks vitamin B12 (VB12) absorption in the terminal ileum. Thus, regular monitoring of serum VB12 concentration is recommended during long-term metformin treatment. Imeglimin is a small molecule synthesized from metformin via a single step chemical reaction, but whether it blocks VB12 absorption like metformin remains unclear. In this non-interventional, retrospective longitudinal study, we investigated the effects of 24 weeks of imeglimin treatment on microalbuminuria as an initial marker of albuminuria and serum VB12 concentration in Japanese patients with type 2 diabetes and microalbuminuria.
Methods: Twenty-one patients (14 men, seven women) with type 2 diabetes and microalbuminuria (30 ≤ urinary albumin-to-creatinine ratio (UACR) < 300 mg/gCre) aged 30 to 80 years (mean ± standard error (SE), 65.2 ± 2.4) were enrolled. Oral imeglimin was started at 2,000 mg daily and continued at the same dose for 24 weeks. Other anti-diabetic agents were continued at their existing dosages. Laboratory examination was performed at baseline, 12 weeks, and 24 weeks, and the effect of imeglimin on glycemic control, UACR, and serum VB12 concentration were assessed.
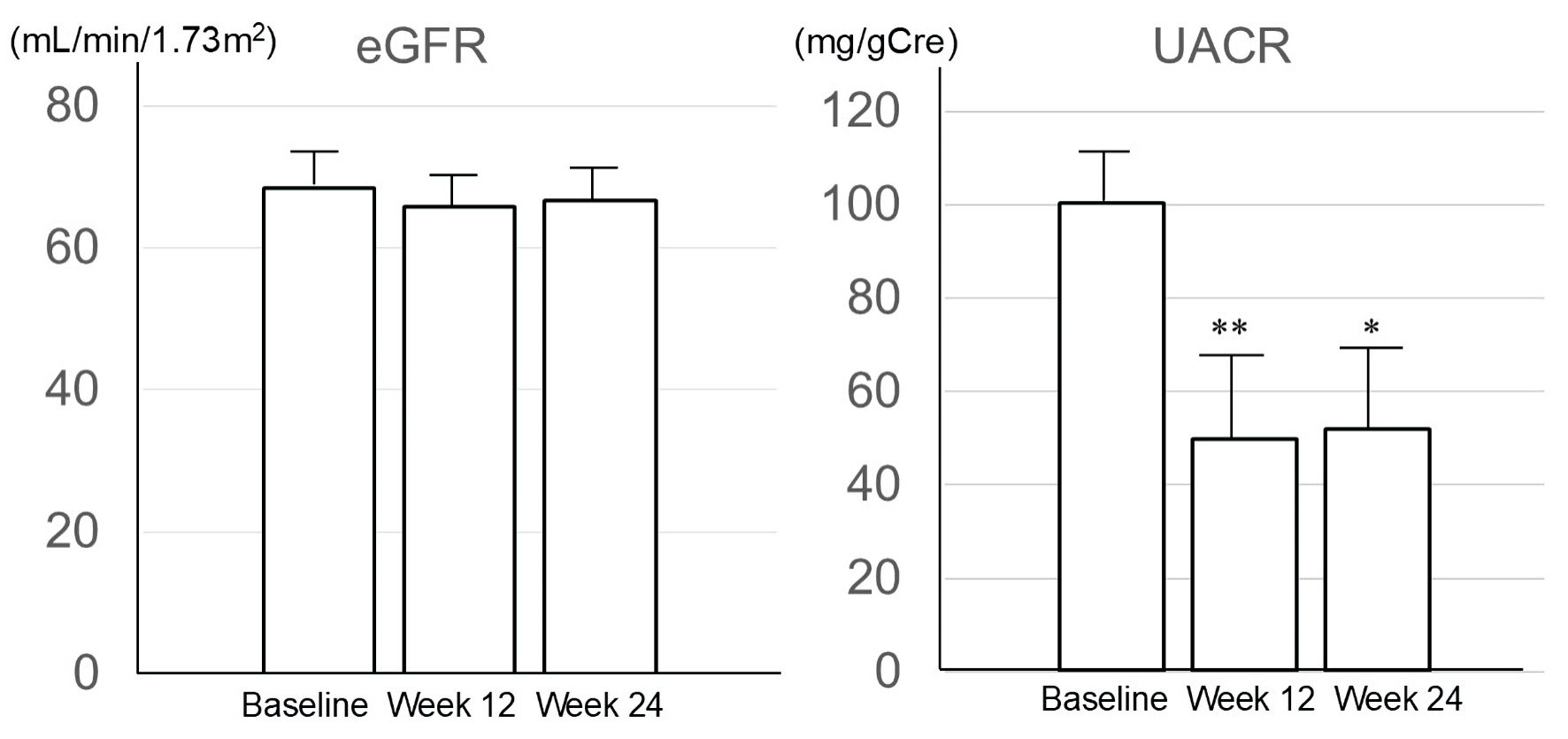
Results: Mean (± SE) hemoglobin A1c decreased by 0.97±0.17% from baseline to 24 weeks, and UACR also decreased significantly (100.32 ± 12.73 mg/gCre at baseline; 49.74 ± 19.32 mg/gCre at 12 weeks; 51.78 ± 21.59 mg/gCre at 24 weeks). Overall, serum VB12 concentration showed no significant change at 24 weeks. However, subgroup analysis showed a decrease in serum VB12 concentration at 12 and 24 weeks in patients receiving concomitant metformin (323.25 ± 51.75 pg/mL at baseline; 262.75 ± 40.96 pg/mL at 12 weeks; 249.75 ± 41.28 pg/mL at 24 weeks), but not in those not receiving metformin.
Conclusions: Twenty-four weeks of imeglimin treatment ameliorated glycemic control and microalbuminuria, but it decreased serum VB12 concentration in patients receiving metformin. Thus, a large-scale prospective study should be needed to confirm the results and clarify the clinical relevance in patients with type 2 diabetes.
Published
Issue
Section
License
Copyright (c) 2025 The authors

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.